Background
High-risk (HR) newly-diagnosed multiple myeloma (NDMM) has poor outcomes with standard first-line therapies, even in transplant-eligible (TE) patients (pts). A CAR-T therapy with high efficacy and manageable safety profile would be a potential solution to this significant unmet need. GC012F is an autologous B cell maturation antigen (BCMA) and CD19 dual-targeting CAR-T cells therapy developed on the novel FasTCAR-T enabling next-day manufacturing platform [J Clin Oncol 41, 2023 (suppl; abstr 8005)]. The phase I single-arm study has been conducted in frontline setting for TE high-risk NDMM pts to characterize the safety and feasibility of GC012F CAR-T cell therapy (NCT04935580). The data was presented at ASH 2022 for initial 13 pts (Blood (2022) 140 (Supplement 1): 889-890.). Here we present updated data with longer follow up and 9 additional pts treated (total N=22) in this study.
Methods
This is a single arm, open-label phase I investigator-initiated study (NCT04935580). TE NDMM pts, aged between 18-70, and with one or more of the following features were considered eligible for the study: R-ISS-II or-III; del17p, t (4;14), t (14;16), or 1q21amp ≥ 4 copies; extramedullary disease (EM); IgD or IgE subtype; LDH > the upper limit of normal; or any of the high-risk definition of mSMART3.0.
As of the data cutoff date, 22 evaluable pts (median age 59, range 43-69) are reported here. The median time from diagnosis to infusion was 100 days (range 63-152). All patients had one or more high-risk features including 91% R-ISS stage II or III, 55% with EM, 32% 1q21≥4 copies, and 9% IgD type. Of the 22 pts, 21 pts received 2 cycles induction therapy of bortezomib, lenalidomide and dexamethasone (VRd), and one patient received 1 cycle bortezomib, epirubicin, and dexamethasone (PAD) and 1 cycle VRd prior to the infusion. GC012F was administered as a single infusion at 3 doses levels (DL) of 1x10 5/kg (n=1), 2x10 5/kg (n=4), or 3x10 5/kg (n=17), after a standard 3-day lymphodepletion consisting of cyclophosphamide and fludarabine .
Results
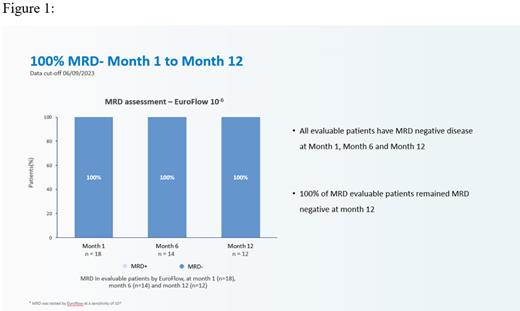
As of June 9 th, 2023 data cutoff, 22 patients were enrolled and evaluable for safety and efficacy. Median follow-up was 13.6 months (range 2.1-23.9 months). Overall response rate (ORR) was 100% and stringent complete response (sCR) rate was 95.5%. All treated pts (100%) across all dose levels achieved minimal residual disease (MRD) negativity assessed by Euroflow (sensitivity of 10 -6). All evaluable pts achieved MRD negativity at Month 1, and maintained MRD- at landmark analysis of Month 6 and Month 12. Median duration of response (DOR) and progression-free survival (PFS) were not reached. Only 6 pts (27%) experience low-grade cytokine release syndrome (CRS), including 23% grade 1 (n=5) and 4% grade 2 (n=1). No treatment-related grade ≥3 CRS, nor ICANS of any grade, and nor deaths occurred in the study. Robust CAR T-cell expansion was observed in all pts; the median peak expansion (Cmax) was 62,131 (range: 8,754-331,159) copies /μg DNA with a median Tmax of 10 days (range 9-14 d).
Conclusion
Consistent with the previous RRMM cohort treated with GC012F, initial data from this phase I study demonstrated that BCMA-CD19 dual-targeting FasTCAR-T GC012F resulted in deep and durable response in transplant-eligible newly-diagnosed high-risk pts with a very favorable safety profile. All three dose groups achieved 100% MRD negativity and 100% ORR and sCR. The promising preliminary results achieved with GC012F demonstrate potential of CAR-T therapy in newly-diagnosed MM pts. Further research with larger patient population and longer follow-up shall bring the hope to this unmet medical need.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal